- Cell Line Development Platform
- CMC
- Protein Production
- GMP Virus Manufacturing
- Microbial & Yeast Production
- GMP Cell Therapy Preparation
-
Cell Lines and Culture Media
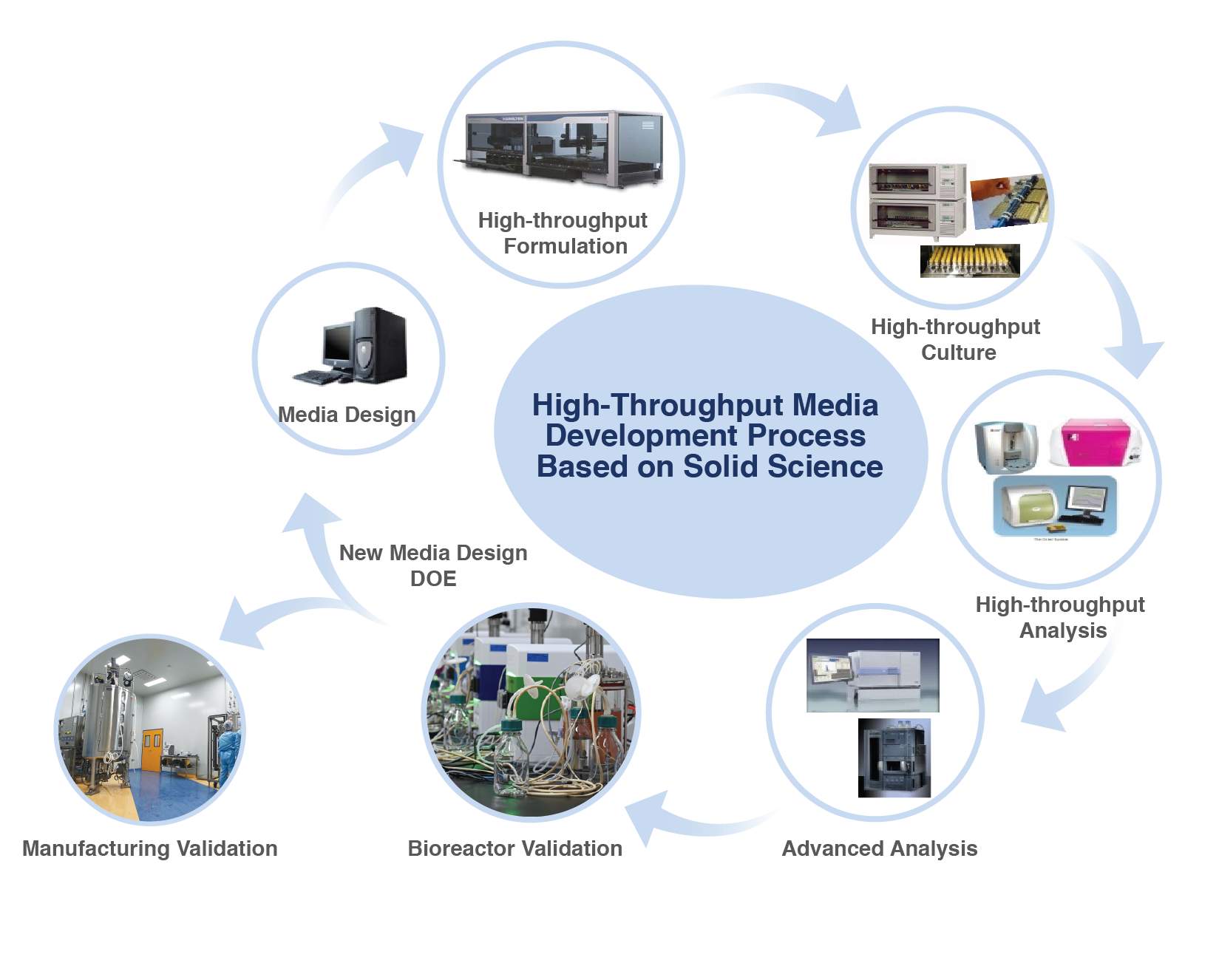
BioInno Bioscience has a large number of culture media formulas with independent intellectual property rights and has experience in commercial development and production. In terms of serum-free cell culture media development, our serum-free cell culture media development system and platform are in an international leading position and can meet the needs of customers at all stages from preclinical to commercial. We use Solentim VIPs and SONY flow cytometry to ensure better selection and to accelerate the optimization of clones.
Media Research & Production
Customized Media Development
- Proprietary media formulations
- International leading metabolic engineering and metabolic modeling tools
- High-throughput platforms (DOE + Automation)
Contract Media Manufacturing
- Non-GMP powder media manufacturing (1-50Kg/Batch)
- GMP powder media production (500kg/Batch, annual output > 350 tons)
- GMP and non-GMP liquid media production (500 and
2000L batches, annually over 480,000L)
Catalog Media Products
- CHO, HEK293 and other CD media and feeds
- DMEM, F12 and other basic media
- Insect cells, Vero cells, BHK21 and other media
- T cell and stem cell media
Cell Line Development Platform
BioInno Bioscience can develop stable and high-yield cell lines, which can reduce the complexity of downstream purification processes and ensure the production of high-quality antibody drugs.
Vector construction and transformation, and packaging process optimization of common viruses (including AAV, adenovirus, lentivirus and baculovirus).
A variety of host cells can be selected, including CHO-K1, CHO-DG44, HEK 293 and other cell lines with full commercial authorization.
Well-developed transient and stable transformation systems provide protein for customer early research.
Our efficient and robust screening platform can achieve within 2.5 months the top clone with expression above 5g/L.
The ability to construct and prepare cell banks (MCB & WCB) in accordance with European, US and China GMP regulations.
-
-
-
-
-
-
-
-
-
-
-
Process development and biopharmaceutical research (CMC) services
Gene sequence confirmation
Molecular sequence design and optimization (including humanization and codon optimization)
Screening and optimization of primer sequences
Molecular structure screening and optimization
-
-
-
Development and construction of cell lines
Vector construction and transformation, and packaging process optimization for common viruses (including AAV, adenovirus, lentivirus and baculovirus).
A variety of host cells may be selected, including CHO-K1, CHO-DG44, HEK 293 and other cell lines with full commercial authorization.
Well-developed transient and stable transformation systems provide protein for customers' early research.
Efficient and robust screening platform can achieve within 2.5 months the top clone with expression amount above 5g / L.
The ability to construct and prepare cell banks (MCB & WCB) in accordance with European, US and China GMP regulations.
-
-
-
-
-
Upstream process
Our industry-leading cell culture development platform, combined with independently developed culture media manufacturing system, can quickly complete the optimization of 3 – 50L cell culture processes, and maximize the yield, quality, reliability, scalability and production consistency of products.
Process development of multiple cell culture processes such as fed-batch and perfusion.
Process scale up to 50L in development.
Process optimization, process characterization and process verification in later clinical stages.
-
-
-
-
Downstream Process
Mature antibody purification platform processes and extensive experience in multi-type protein purification.
Laboratory scale protein purification from micrograms to 100g.
Product types include monoclonal antibodies, bispecific antibodies, Fc fusion proteins, ADCs, recombinant proteins, and others, through the development of chromatography processes (AC, AEX, CEX, HIC, Multi-Mode, etc.), filtration processes (depth filtration, nanofiltration, ultrafiltration, etc.) and other processes (centrifugation, salting out, etc.). A set of stable and sustainable production processes can be developed in 2 – 4 months, with an overall yield reaching 70% or higher.
-
-
-
Formulation Process Development
Development includes DOE optimization testing on formulation buffers, pH, excipients, amino acids, etc.
Screening of formulations and studying compatibility of packaging materials.
Quickly and efficiently select the appropriate formulation and evaluate the stability of the product.
-
-
-
Quality Research
Provide quality analyses required for process development and formulation development.
Protein process characterization.
Release and stability testing to support IND and BLA applications.
Compatibility studies (method development and optimization, method qualification and validation, and method).
-
-
-
-
-
Protein Production
BioInno protein production platforms primarily adopt leading global brands (such as Cytiva, Sartorius and ABEC) single-use bioreactors, with multiple parallel disposable production lines that can support the parallel production of multiple batches of clinical materials at the same time. At present we have the world's first disposable 6000L bioreactor and matching stock solution production line.
Product Lines
- 6000L Single-Use Bioreactor and Production Line, 2 Lines
- 2000L Single-Use Bioreactor and Production Line, 2 Lines
- 1000L Single-Use Bioreactor and Production Line, 1 Line
- 250L Single-Use Bioreactor and Production Line, 1 Line
- 500L Single-Use Bioreactor and Production Line, 2 Fed-Batch Lines,
2 Fed-batch + Perfusion Lines
- 200L Single-Use Bioreactor and Production Line, 2 Fed-Batch Lines,
2 Fed-batch + Perfusion Lines
Advantages
All single-use bioreactors, including fed-batch and perfusion culture, are leading global brands.
Single use technology is commonly used in liquid preparation, liquid storage, sterilization and filtration.
Recombinant proteins can be harvested by continuous flow centrifugation or depth filtration, as needed for different processes.
All process testing instruments are leading global brands.
Independent air-conditioned areas, with the operation area with potential virus risk physically isolated from the operation area reduce potential virus contamination risk.
-
-
-
-
-
-
GMP Virus Manufacturing Platform
BioInno Bioscience can provide preparation services for viruses (such as lentivirus LV, adeno-associated virus AAV, oncolytic herpes simplex virus HSV and adenovirus AV), and has 100L and 500L production lines.
- Vector identification
- Establishment of cell bank (HEK 293 cell bank)
- Process development
- Process amplification
- Non-clinical research level virus production to clinical and commercial GMP productions
- Quality testing
-
Microbial & Yeast Production
BioInno Bioscience has a service platform for the development and production of microorganisms and yeast, including plasmids, vectors, recombinant proteins, polypeptides, nanoantibodies, cytokines, mRNA and other process development and production projects, so as to meet the different business needs of our clients.
Plasmid Service
Vector plasmid construction
Strain construction
Clone screening
Strain library establishment and stability study
Development, optimization and validation of analytical methods
Process development optimization and validation
Process transfer and scale up
Non-clinical research level virus production through clinical and commercial GMP productions
All-disposable technologies can be used in the upstream (independent production lines with 50L and 200L fermentation tanks).
-
-
-
-
-
-
-
-
-
Service Advantages
The overall environmental cleanliness level in virus manufacturing areas is Grade C.
Single-use technology is used in solution preparation, solution storage, and sterile filtration.
Upstream is a complete suspension culture protocol, using single-use technology (independent manufacturing lines, with 100L and 500L ABEC single-use bioreactors).
Depth filtration system and single-use continuous flow centrifuge technology can be used for harvest.
Manufacturing line downstream equipment (chromatography system, chromatography column, ultrafiltration system and hollow fiber system) covers a scale of 100 – 500L and is in compliance with GMP requirements.
-
-
-
-
-
-
-
BioInno provides CAR-T, UCAR-T, CAR-NK, TCR-T and other cell therapy CDMO services. We offer advanced cGMP facilities with an automated and closed culture approach for reliable clinical and commercial production that meets the transfer and commercial scale up needs of various production processes.
- Process development and optimization
- Production of GMP standard cell therapy products
- Development and validation of analytical methods
- QC inspection and release
- Stability study
- IND filling support
GMP Cell Therapy Preparation Platform